Medical Devices & Components for GS1 UDI
Manufacturers of medical devices use laser marking on medical devices because of the mark clarity, permanency (no added paints or chemicals), and its ability to survive repeated sterilization.
The medical annealing process is approved by the FDA, so instrument manufacturers have one less hurdle in the product approval process. The medical laser annealing process can produce a dark, smooth mark on stainless steel, titanium and most other metals so the laser marking does not trap blood or tissue material and thus simplifies cleaning and sterilization. Delrin, Teflon, PEEK, and ABS are examples of medical plastics that are commonly marked by the laser plastics engraving process.
Laser Impressions Inc. can capably meet your GS1 UDI (Unique Device Identification) FDA implementation requirements for medical devices requiring Data Matrix (datamatrix) using direct part marking (DPM).
Laser Impressions Inc. processes and procedures are structured under ISO 9001:2008 and ISO 13485:2003 to ensure quality and repeatability for your laser marking and laser engraving requirements.
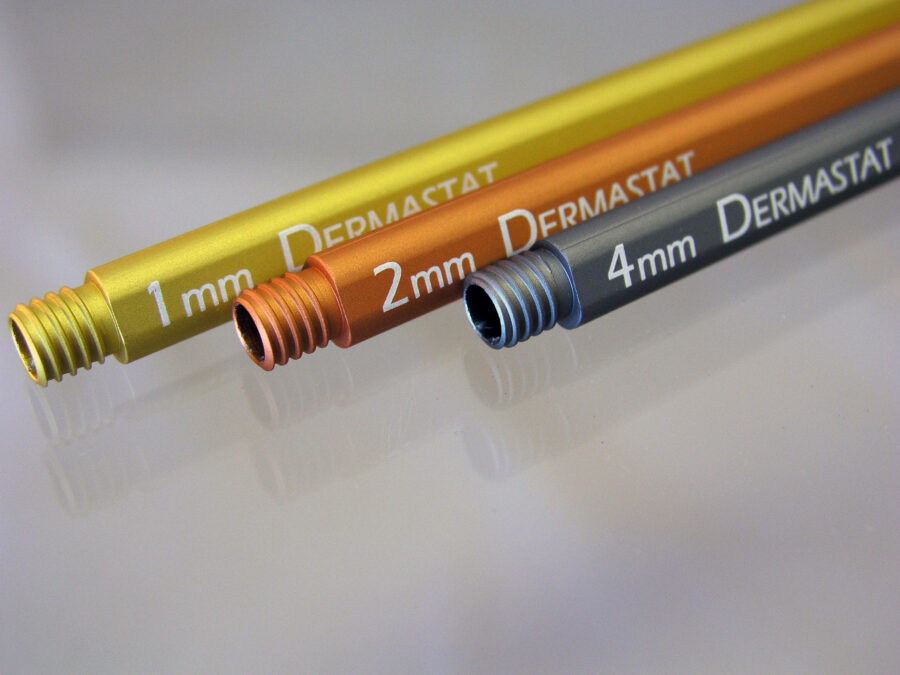



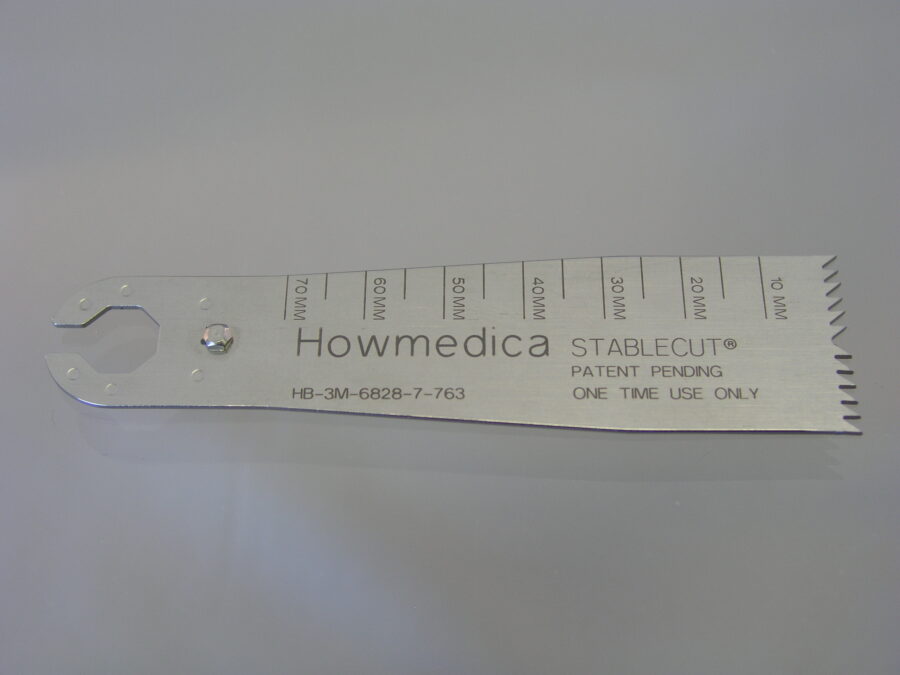

Discover what we can do for you…




